At a constant temperature, only a certain amount of gas adsorption can exist on the solid surface corresponding to a certain adsorbate pressure. The adsorption isotherm can be obtained by measuring the corresponding adsorption amount under a series of relative pressures. The adsorption isotherm is the basic data for the adsorption phenomenon and the surface and pores of the solid. The surface and pore properties can be studied to calculate the specific surface area and pore size distribution.
There are six adsorption isotherms (Figure 1). The first five have been assigned a type number, and the sixth one has been added in recent years. The shape of the adsorption isotherm is directly related to the size and the number of holes.
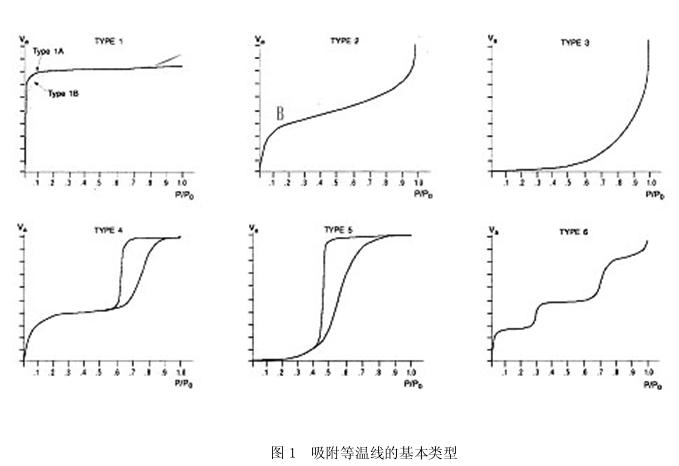
Type I isotherm: The Langmuir isotherm corresponds to the reversible adsorption process of the Lange's monolayer. It is a narrow pore for adsorption, and for micropores, it can be said to be the result of volume filling. The outer surface area of ​​the sample is much smaller than the inner surface area of ​​the pore, and the adsorption capacity is controlled by the pore volume. The pores of the platform turning point corresponding to the adsorbent are completely filled with the condensed liquid. Such isotherms occur in microporous silica gel, zeolite, carbon molecular sieve, and the like.
When such an isotherm is close to the saturated vapor pressure, due to the existence of a gap between the particles, adsorption similar to that of the large hole occurs, and the isotherm rises rapidly.
Type II isotherms: S-type isotherms correspond to a single multilayer reversible adsorption process that occurs freely on non-porous solid surfaces or macroporous solids. There is an inflection point B at the low P/P, which is the first steep portion of the isotherm, which indicates the saturated adsorption amount of the monolayer, which is equivalent to the completion of the monolayer adsorption. As the relative pressure increases, the second layer begins to form, and at saturated vapor pressure, the number of adsorbed layers is infinite. This type of isotherm is often encountered when the adsorbent pore size is greater than 20 nm. Its solid pore size has no upper limit. In the low P/P region, the curve is convex upward or convex downward, reflecting the strong or weak interaction of the adsorbate with the adsorbent.
Type III isotherm: convex downwards over the entire pressure range, curve without inflection point B occurs when a multi-molecular layer occurs on the sputum surface, or the adsorption interaction between the solid and the adsorbate is less than the interaction between the adsorbates Types of. For example, water vapor is adsorbed on the graphite surface or adsorbed on the non-porous metal oxide subjected to the hydrophobic treatment. The amount of adsorption in the low pressure zone is small, and the point B does not appear, indicating that the force between the adsorbent and the adsorbate is rather weak. The higher the relative pressure, the more the amount of adsorption, showing a pore filling. Some systems (such as the adsorption of nitrogen on various polymers) show a gradually curved isotherm with no identifiable point B. In this case, the interaction between the adsorbent and the adsorbate is relatively weak.
Type IV isotherm:
The low P/P zone curve is convex upwards, similar to the Type II isotherm. In the higher P/P region, capillary adsorption occurs in the adsorbate, and the isotherm rises rapidly. When all the pores have agglomerated, the adsorption occurs only on the outer surface much smaller than the inner surface area, and the curve is flat. When the relative pressure 1 is close, it is adsorbed on the large hole and the curve rises. Due to capillary condensation, hysteresis can be observed in this region, that is, the isotherm obtained during desorption does not coincide with the isotherm obtained during adsorption, and the desorption isotherm is above the adsorption isotherm, causing adsorption hysteresis (adsorption) Hysteresis), showing a hysteresis loop. This adsorption hysteresis is related to the shape and size of the pores. Therefore, the size and distribution of the pores can be known by analyzing the adsorption and desorption isotherms.
Type IV isotherms are the most common adsorption behavior of mesoporous solids, and most industrial catalysts are IV.
Type isotherm. The hysteresis loop is related to the secondary process of capillary condensation.
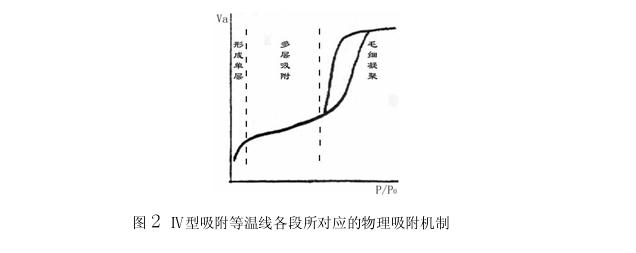
The physical adsorption mechanism corresponding to each section of the IV adsorption isotherm:
The first stage: first form a single layer of adsorption, the inflection point B indicates the single layer of saturated adsorption amount of the second stage: the first stage of multi-layer adsorption: capillary condensation, wherein the starting point of the hysteresis ring indicates that the smallest capillary begins to agglomerate; The end point indicates that the largest pore is filled with condensed liquid; the platform appears after the hysteresis loop, indicating that the entire system is filled with condensed liquid, and the amount of adsorption is no longer increased, which means that the pores in the system have a certain upper limit.
V-type isotherm: less common, and difficult to explain, although reflecting the weak type III isotherm between the adsorbent and the adsorbate, it also shows pore filling in the high pressure zone. Sometimes there are capillary agglomerations and hysteresis loops in the higher P/P regions.
Type VI isotherm: Also known as stepped isotherm. It is a special type of isotherm that reflects the results of harmonic multi-layer adsorption on a uniform surface of a solid (such as the adsorption of ruthenium on certain clean metal surfaces). In fact, the surface of the solid, especially the surface of the catalyst, is mostly non-uniform, so it is difficult to encounter this situation.
The shape of the isotherm is closely related to the nature of the adsorbate and the adsorbent, so the study of the isotherm can obtain information about the properties of the adsorbent and adsorbate. For example, the specific surface area of ​​the solid can be calculated from the isotherm of type II or IV; the isotherm of type IV is characterized by medium pores (hole width between 2 and 50 nm), with inflection point B and hysteresis loop, and thus is used for medium-range pores. The hole distribution is calculated.
Cell Phone Case, Mobile Phone Covers, Clear Phone Case, Mobile Phone Case, TPU Phone Case, Silicone Phone Case
Shenzhen Jianjiantong Technology Co., Ltd. , https://www.mowang-dg.com